What are the key points about radioactive decay and half-life?
To understand why some nucleiNuclei is the plural of nucleus. The nucleus is the central part of an atom. It contains protons and neutrons, and has most of the mass of the atom. are radioactivityThe process where certain material decay and emit one of 3 different types of radiation (alpha, beta or gamma). .
What is alpha radiationA type of ionising radiation consisting of 2 protons and 2 neutrons., beta radiationA type of ionising radiation consisting of a single electron. and radiation - and what are the differences between them?
To understand nuclear decay equations.
How to measure the activity of radioactive sources.
How to calculate half-lifeThe half-life of a radioactive source is the time taken for its activity to fall to half of the original activity. .
What is radioactivity?
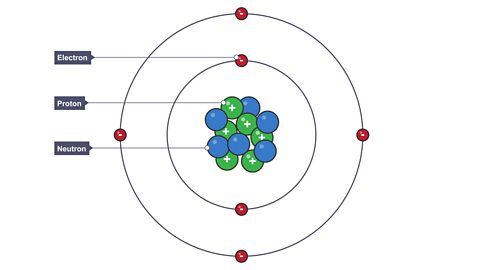
Stable and unstable nuclei
Radioactivity is caused by the nucleus of an isotopeAtoms of an element with the same number of protons but different numbers of neutrons. being unstable.
The ratioA ratio shows how much of one thing there is compared to another. of protonSubatomic particle with a positive charge and a relative mass of 1. The relative charge of a proton is +1. and neutronUncharged subatomic particle, with a relative mass of 1. The relative charge of a neutron is 0 (neutral). in a nucleusThe central part of an atom. It contains protons and neutrons, and has most of the mass of the atom. The plural of nucleus is nuclei. determines whether a nucleus will be stable or unstable.
Too many neutrons or protons can upset this balance making the nucleus unstable.
Carbon-12 is stable and has six protons and six neutrons.
However as the number of protons increases, more neutrons are needed to keep the nucleus stable.
For example lead, lead-206 has 82 protons and has 124 neutrons.
Nuclei with too many, or too few, neutrons do exist naturally but are unstable and will disintegrate (or decay) by emitting radiation.
This is called radioactive decay.
It is important to realise that radioactive nuclei disintegrate:
spontaneously
and randomly.
Spontaneously means that the process of radioactive decay can not be speeded up or slowed down by physical factors, e.g. temperature or pressure etc.
Random means that we cannot predict when an individual unstable nucleus will decay.
Key facts
Some nuclei are unstable.
They disintegrate spontaneousIn relation to radioactive decay, this means it is a process which cannot be sped up or slowed down by physical conditions (e.g. temperature and pressure). and randomIn relation to radioactive decay, this means it is impossible to predict when it will happen (for a particular nucleus). emitting radiation.
Such nuclei are described as radioactive.
What is ionising radiation?
The radiation emitted from unstable nuclei is called ionising radiation because as it passes through matterSub-atomic particles and anything made from them, such as atoms and molecules, are matter. Energy and forces are not matter. it can dislodge outer electronA subatomic particle with relative mass of ¹⁄₁₈₄₀. The relative charge of an electron is -1. from atoms causing them to become ionElectrically charged particle, formed when an atom gains or loses electrons..
What are the different types of radioactive decay?
An unstable nucleus can decay by emitting an alpha particle, a beta particle, or a gamma ray.
What is alpha radiation?
An unstable nucleus can emit a ‘package’ of two protons and two neutrons, called an alpha particle, to become more stable.
Alpha radiation is made up of a stream of alpha particles emitted from unstable nuclei within a radioactive substance.
An alpha particle is also a helium-4 nucleus.
It is written as
\(_{2}^{4}\textrm{He}\) and is also sometimes written as \(_{2}^{4}\alpha\).
Alpha decay causes the mass numberThe total number of protons and neutrons found in the nucleus of an atom. of the nucleus to decrease by four and the atomic numberThe number of protons in the nucleus of an atom. of the nucleus to decrease by two.
Image caption, 1. An unstable nucleus can decay by the emitting an alpha particle to become more stable.
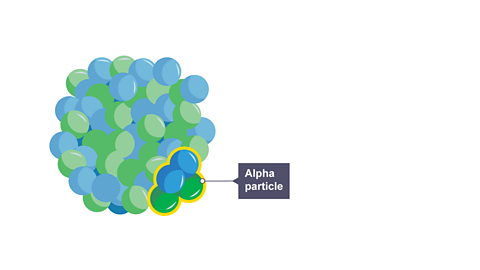
Image caption, 2. An alpha particle is a “ball” of two protons and two neutrons.
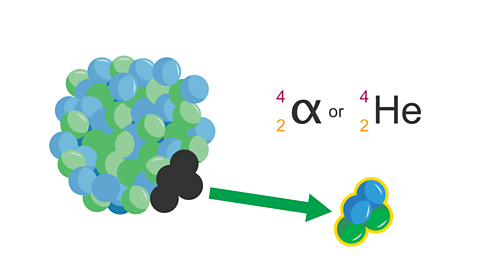
Image caption, 3. The Alpha particle is ejected from the nucleus. The alpha particle is sometimes called a helium nucleus.
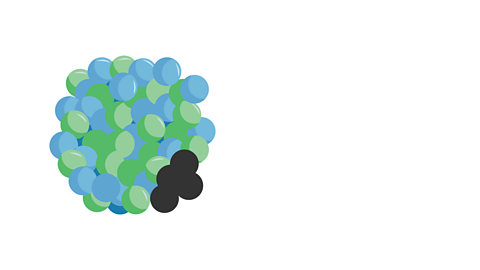
Image caption, 4. A new element is formed as the nucleus now has less protons and neutrons. The atomic number of the new nucleus has decreased by 2 (as it has lost 2 protons). The mass number has decreased by 4 (as it has lost 2 neutrons and 2 protons).
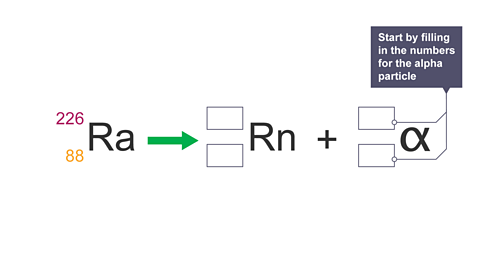
Image caption, 5. Alpha decay equation: to complete the decay equation in the exam, start by filling in the numbers for the alpha particle.
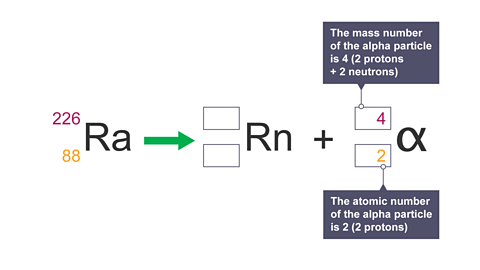
Image caption, 6. Calculate the mass number and the atomic number by adding up the protons and neutrons.
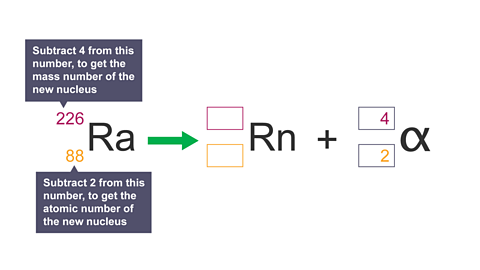
Image caption, 7. Calculate the atomic number and mass number of the new nucleus.
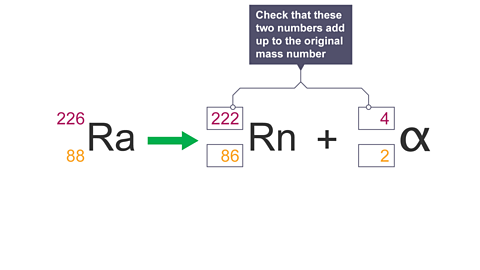
Image caption, 8. Check these numbers add up to the original mass number.
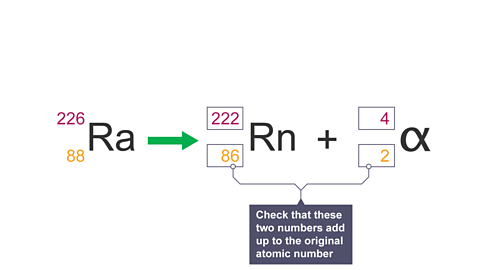
Image caption, 9. Check these numbers add up to the original atomic number.
1 of 9
What is the alpha decay equation?
\(_{Z}^{A}\textrm{X} \rightarrow _{Z-2}^{A-4}\textrm{Y} +\ _{2}^{4}\textrm{He}\) or \(_{2}^{4}\textrm{α}\)
Note that the mass numbers and atomic numbers are equal (i.e. they balance) on both sides of the equation.
Example
Alpha decay of Uranium-238
\(_{92}^{238}\textrm{U} \rightarrow _{90}^{234}\textrm{Th} +_{2}^{4}\textrm{He}\)
What is beta radiation?
An unstable nucleus can emit a fast-moving electron called a beta (β) particle, to become more stable.
Beta radiation is made up of a stream of beta particles emitted from unstable nuclei within a radioactive substance.
Beta radiation is normally emitted from unstable nuclei in which the number of neutrons is much larger than the number of protons.
A beta particle has a relative massThe relative mass is the number of times heavier a particle is, compared to another. that can be considered to be zero, so its mass number is zero, and as the beta particle is an electron, it can be written as: \(_{-1}^{~0}\textrm{e}\)
Sometimes, it is also written as: \(_{-1}^{~0}\beta\).
Image caption, 1. An unstable nucleus that has too many neutrons can decay by the emitting a beta particle to become more stable.
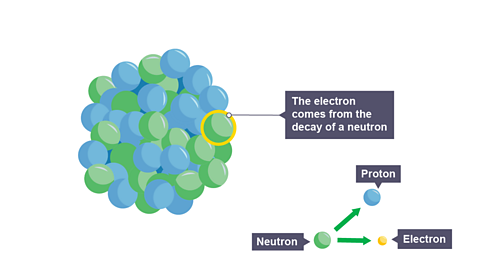
Image caption, 2. The neutron splits into a proton and an electron (beta particle). A beta particle is really an electron ejected from the nucleus. BUT - there are no electrons in the nucleus.
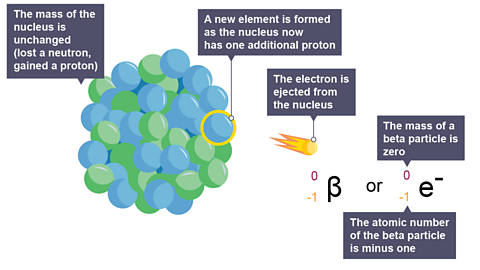
Image caption, 3. The electron is ejected from the nucleus. This is the beta particle that can be detected.
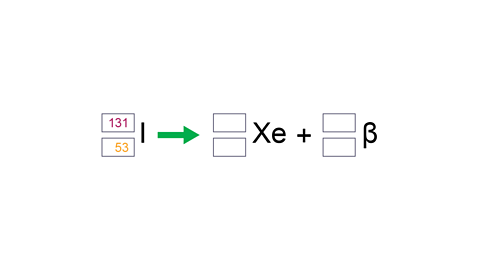
Image caption, 4. Beta decay equation: to complete the decay equation in the exam, start by filling in the numbers for the beta particle.
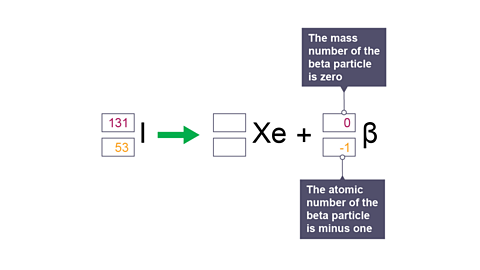
Image caption, 5. The mass number of the beta particle is zero. The atomic number of the beta particle is minus one.
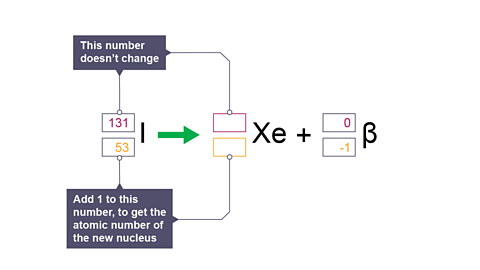
Image caption, 6. Decay equation: how to calculate the atomic number of the new nucleus.
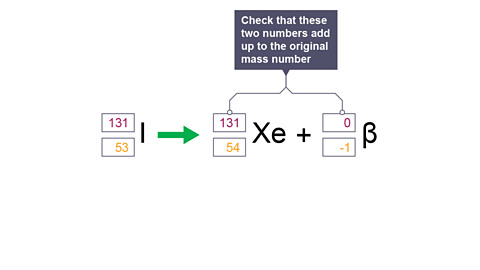
Image caption, 7. Check that these two numbers add up to the original mass number.
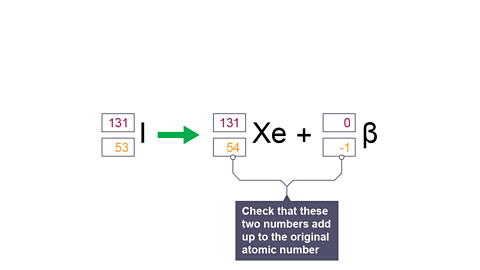
Image caption, 8. Check that these two numbers equal the original atomic number.
1 of 8
Key fact
The beta particle is an electron but it has come from the nucleus, not the shellAn energy level around the nucleus where electrons can be found orbiting. of the atom.
Electrons are not normally found in the nucleus but, in an unstable nucleus, a neutron can split into a positive proton and a negative electron.
The proton remains inside the nucleus, but the electron is ejected at high speed.
This is called beta decay.
Beta decay causes the atomic numberThe number of protons in the nucleus of an atom. of the nucleus to increase by one (because there is an extra proton) but the mass numberThe total number of protons and neutrons found in the nucleus of an atom. remains the same (because the total number of protons and neutrons remains unchanged).
What is the equation for beta decay?
\(_{Z}^{A}\textrm{X} \rightarrow _{Z+1}^{A}\textrm{Y} + _{-1}^{0}\textrm{e}\) or \(_{-1}^{~0}\beta\)
Example
Beta decay of carbon-14
\(_{6}^{14}\textrm{C} \rightarrow_{7}^{14}\textrm{N} +\ _{-1}^{0}\textrm{e}\)
What is gamma radiation?
Gamma radiation does not consist of particles but as short wavelength, high energy electromagnetic radiation emitted from unstable nuclei.
It is normally emitted alongside alpha radiationA type of ionising radiation consisting of 2 protons and 2 neutrons. or beta radiationA type of ionising radiation consisting of a single electron. radiation.
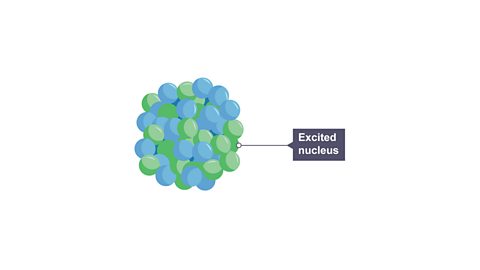
Image caption, 1. After emitting an alpha or beta particle, the nucleus will often still be ‘excited’ and will need to lose energy.
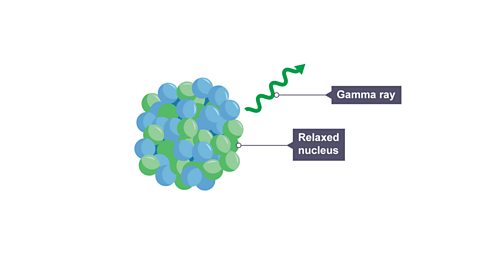
Image caption, 2. It does this by emitting a high energy electromagnetic wave called a gamma ray. The gamma ray is a “burst” of energy. It has no mass. It has no charge. No new element is formed as the nucleus still has the same number of protons.
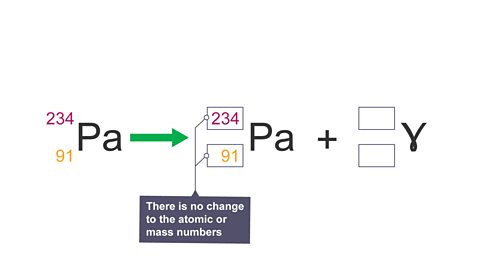
Image caption, 3. Gamma decay equation: there is no change to the atomic or mass numbers.
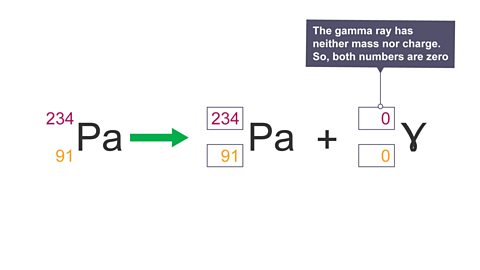
Image caption, 4. Gamma decay equation: the gamma ray has neither mass nor charge. So, both numbers are zero.
1 of 4
Gamma ray emission causes no change in the number of particles in the nucleus meaning both the atomic number and mass number remain the same.
It can be written as γ or \(_{0}^{0}\textrm{γ}\)
What is the gamma decay equation?
\(_{X}^{A}\textrm{X}\rightarrow_{Z}^{A}\textrm{Y}+_{0}^{0}\textrm{γ}\) or γ
Example
Beta and gamma decay of cobalt-60
\(_{27}^{60}\textrm{Co}\rightarrow_{28}^{60}\textrm{Ni}+_{-1}^{0}\textrm{e}+_{0}^{0}\textrm{γ}\)
What are the properties of nuclear radiations?
The different types of radiation are often compared in terms of their penetrating powerThe power of the radiation that demonstrates how far into a material the radiation will go., their ioniseTo ionise is to convert an uncharged atom into a charged particle by adding or removing electrons. and how far they can travel in the air.
| Symbol | Stopped by | Penetration power | Ionising power | Range in air | |
|---|---|---|---|---|---|
| Alpha | α | Skin/paper | Low | High | 4-6 centimetre (cm) |
| Beta | β | 5 mm aluminium | Medium | Low | ≈ 1 metre (m) |
| Gamma | γ | Reduced by thick lead/concrete | High | Very low | > 1 kilometre (km) |
All types of radioactive decay can be detected by a Geiger-Muller tubeDevice used to detect and measure the quantity of ionising radiation in an area., or G-M tube.
What are the key points about the different radiations?
Alpha radiation
Alpha particles are helium nucleiNuclei is the plural of nucleus. The nucleus is the central part of an atom. It contains protons and neutrons, and has most of the mass of the atom. consisting of two protonSubatomic particle with a positive charge and a relative mass of 1. The relative charge of a proton is +1. and two neutronUncharged subatomic particle, with a relative mass of 1. The relative charge of a neutron is 0 (neutral). emitted from unstable nuclei \(_{2}^{4}\textrm{α}\)
Alpha radiation is stopped by a few (4-6) centimetres of air or a thin sheet of paper.
Alpha decay: \(_{Z}^{A}\textrm{X}\rightarrow_{Z-2}^{A-4}\textrm{Y}+_{2}^{4}\textrm{He}\) or \(_{2}^{4}\textrm{α}\)
The mass numberThe total number of protons and neutrons found in the nucleus of an atom. of the new nucleus decreases by 4.
The atomic numberThe number of protons in the nucleus of an atom. of the new nucleus decreases by 2.
Alpha particles are relatively heavy and so produce the most ionisationProcess by which electrons can be added or removed from an atom to create an ion..
Beta radiation
Beta particles are fast moving electronA subatomic particle with relative mass of ¹⁄₁₈₄₀. The relative charge of an electron is -1. emitted from an unstable nucleus.
Beta radiation is stopped by several metres of air or a thin (~5 mm) sheet of aluminium \(_{-1}^{~0}\beta\).
Beta decay: \(_{X}^{A}\textrm{X}\rightarrow_{Z+1}^{A}\textrm{Y}+_{-1}^{0}\textrm{e}\) or \(_{-1}^{~0}\beta\)).
The mass number of the new nucleus stays the same.
The atomic number of the new nucleus increases by 1.
Beta particles are much lighter than alpha particles and so produce a great deal less ionisation.
Gamma radiation
Gamma radiation is high energy electromagnetic waves emitted from unstable nuclei.
Gamma radiation easily passes through air, paper, skin and aluminium but can be partly blocked by thick lead or concrete.
Emission of gamma radiation leaves mass number and atomic number unchanged.
Gamma rays produce the least ionisation.
Question
Complete the following decay equation:
\(_{86}^{226}\textrm{Ra}\rightarrow_{Z}^{A}\textrm{Rn}+_{2}^{4}\textrm{He}\)
Mass number 226 = A + 4
A = 226 - 4 = 222
Atomic number 86 = Z + 2
Z = 86 - 2 = 84
\(_{86}^{226}\textrm{Ra}\rightarrow_{84}^{222}\textrm{Rn}+_{2}^{4}\textrm{He}\)
Question
Polonium-210 decays to lead-206. Polonium (Po) has atomic number 84 and lead (Pb) has atomic number 82.
Which type of decay occurs?
\(_{84}^{210}\textrm{Po}\rightarrow_{82}^{206}\textrm{Pb}+_{Z}^{A}\textrm{X}\)
Mass number 210 = 206 + A
A = 210 - 206 = 4
Atomic number 84 = 82 + Z
Z = 84 - 82 = 2
X has mass number 4 and atomic number 2 and so is an alpha particle
\(_{84}^{210}\textrm{Po}\rightarrow_{82}^{206}\textrm{Pb}+_{2}^{4}\textrm{He}\)
How to measure amounts of radiation?
The activity of a radioactive source is the number of decays per second from the unstable nucleiNuclei is the plural of nucleus. The nucleus is the central part of an atom. It contains protons and neutrons, and has most of the mass of the atom. present in the source.
The simplest unit of activity is the Becquerel (Bq).
A source that emits one particle per second has an activity of one Bq.
Activity can also be measured in counts per minute.
Since radioactive decayThis is the disintegration of unstable nuclei by emitting alpha particles, beta particles or gamma rays. Radioactive decay occurs spontaneously and randomly. is a randomIn relation to radioactive decay, this means it is impossible to predict when it will happen (for a particular nucleus). process, it is always good practice to determine the average count rate rather than to measure the counts that occur in just one second or one minute.
Radioactivity can be detected using a Geiger-Muller tube connected to a counter.
When alpha particles, beta particles or gamma rays enter the GM tube the counter clicks and the count is displayed on the screen.
The number of counts per second or per minute is called the count rate.
What is background radiation?
A Geiger-Muller tubeDevice used to detect and measure the quantity of ionising radiation in an area. will detect radiation even when there is no apparent radioactive source present.
This is because radioactive material is found naturally all around us.
Key Fact
Background radiation is the radiation detected when there are no known radioactive sources present.
Examples of natural sources of background radiation:
Radioactive rocks in the ground.
Cosmic rays from space.
How to measure the background radiation
- Remove all known sources of radioactivity from the room.
- Set the counter to zero.
- Switch on and start a stop clock.
- After 20 minutes switch off. Record the count.
- Divide the count by 20 to calculate the count rate per minute.
The background count rate is measured over a period of 20 minutes because of the random nature of radioactive decay.
Dividing by 20 enables the average count rate per minute to be determined.
Background count rate is typically 18 counts per minute which does not present a serious health risk to humans.
Key fact
The background count rate must be subtracted from any other count rate when measuring the activity of a radioactive source. This is known as the corrected count rate.
Corrected count rate = Measured count rate – Background count rate
Question
A scientist measures the background radiation level in their lab and records the following results:
| Experiment number | Counts in 20 mins |
|---|---|
| 1 | 367 |
| 2 | 354 |
| 3 | 359 |
Calculate the average reading for the background radiation level in counts per minute.
Answer
average 20 minute reading = \(\frac{367~+~354~+~359}{3}\) = 1080 counts
counts per minute = \(\frac{1080}{20}\) = 18 cpm
Question
The scientist then measures the activity of a sample of strontium-90 in the same lab.
The average reading is 309 counts per minute.
Calculate the corrected activity of the sample of strontium-90.
Answer
Corrected count rate = Measured count rate – Background count rate
=309- 18
=291
What is half-life?
radioactive decayThis is the disintegration of unstable nuclei by emitting alpha particles, beta particles or gamma rays. Radioactive decay occurs spontaneously and randomly. is a spontaneousIn relation to radioactive decay, this means it is a process which cannot be sped up or slowed down by physical conditions (e.g. temperature and pressure). and randomIn relation to radioactive decay, this means it is impossible to predict when it will happen (for a particular nucleus).process.
A block of radioactive material will contain many trillions of nuclei and not all nuclei are likely to decay at the same time.
As the process of radioactive decay is random, it is impossible to tell when a particular nucleus will decay but given that there are so many of them, it is possible to say that a certain number will decay in a certain time.
This is called the half-life.
Key fact
Half-life is the time taken for the activity of the source to fall to half its original value.
The illustration below shows how a radioactive sample is decaying over time.
From the start of timing it takes two days for the count to halve from 80 Bq down to 40 Bq.
It takes another two days for the count rate to halve again, this time from 40 Bq to 20 Bq.
The half-life of this source is 2 days.
Note that this second two days does not see the count drop to zero, only that it halves again.
A third, two-day period from four days to six days sees the count rate halving again from 20 Bq down to 10 Bq.
This process continues and although the count rate might get very small, it does not drop to zero completely.
The half-life of radioactive carbon-14 is 5,730 years.
If a sample of a tree (for example) contains 64 grams (g) of radioactive carbon after 5,730 years it will contain 32 g, after another 5,730 years that will have halved again to 16 g.
How to set out half-life calculations
Example 1 – If the half-life is known
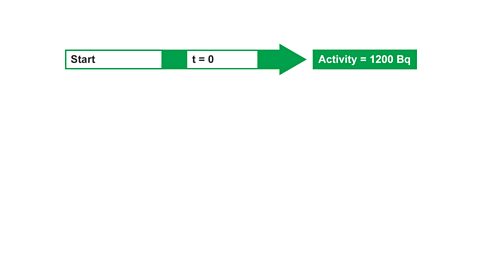
Image caption, 1. The activity of a radioactive source was found to be 1200 Bq. The half-life is 6 hours (t½ = 6 hours). What will the activity be after 24 hours? Start by writing out what you know initially, (at t = 0)...
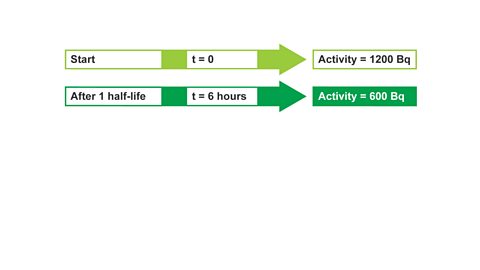
Image caption, 2. Then state what you would know after one half-life. The activity will have halved after one half-life. Repeat the process until the required amount of time has passed.
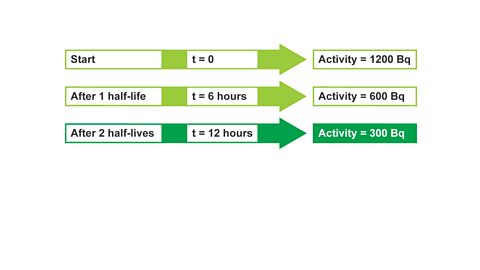
Image caption, 3. After 2 half-lives, t = 12 hours, activity = 300 Bq
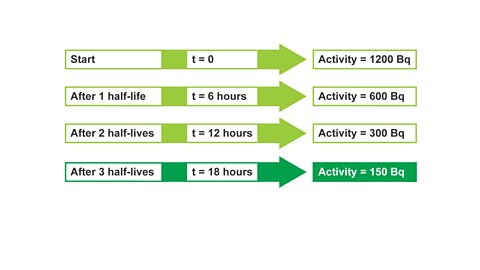
Image caption, 4. After 3 half-lives, t = 18 hours, activity = 150 Bq
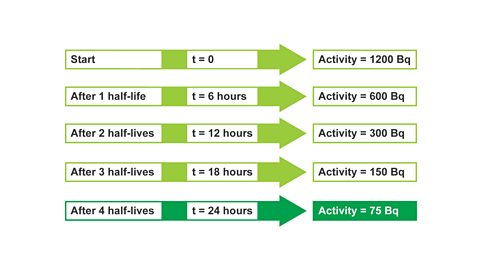
Image caption, 5. After 4 half-lives, t = 24 hours, activity = 75 Bq. The activity has fallen to 75 Bq after 24 hours, (or 4 half-lives).
1 of 5
Example 2 – If the half-life is not known
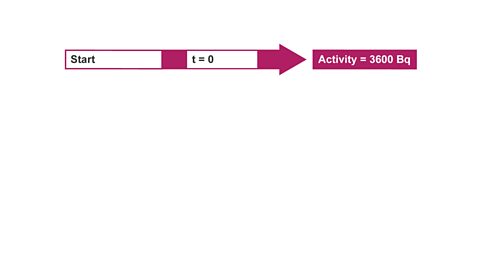
Image caption, 1. The activity of a radioactive source was found to be 3600 Bq. After one hour it had fallen to 450 Bq. Calculate the half-life of the source. Start by writing out what you know initially, (at t = 0). Then state what you would know after one half-life.

Image caption, 2. After one half-life: the activity will have halved after one half-life.
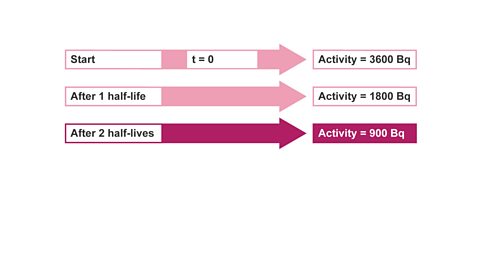
Image caption, 3. Repeat the process until you get the required count rate. So, after two half-lives, activity is now 900 Bq.
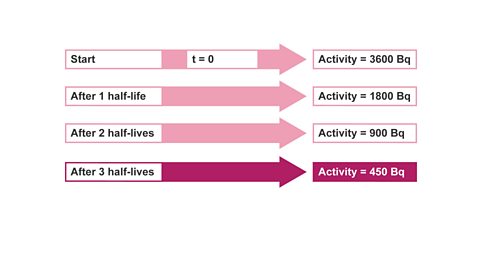
Image caption, 4. After three half-lives, activity is now 450Bq.
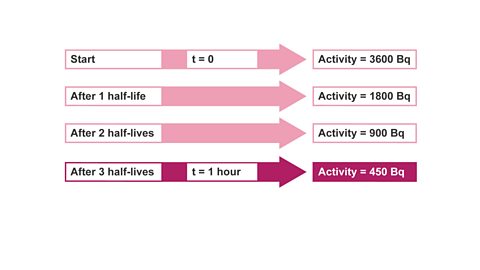
Image caption, 5. This means that 3 half-lives took one hour. 3 t½ = 60 minutes.
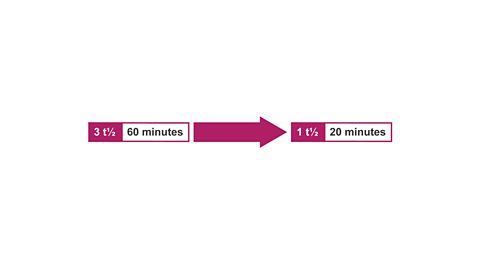
Image caption, 6. Therefore, 1 half-life = 60/3 = 20 minutes.
1 of 6
How to calculate how much of an isotope remains
It should also be possible to state how much of a sample remains or what the activity or count should become after a given length of time.
This could be stated as a fraction, decimal or ratioA ratio shows how much of one thing there is compared to another..
For example the amount of a sample remaining after four half-lives could be expressed as:
a fraction - a \(\frac{\text{1}}{\text{2}}\) of a \(\frac{\text{1}}{\text{2}}\) of a \(\frac{\text{1}}{\text{2}}\) of a \(\frac{\text{1}}{\text{2}}\) remains which is \(\frac{\text{1}}{\text{2}}\) x \(\frac{\text{1}}{\text{2}}\) x \(\frac{\text{1}}{\text{2}}\) x \(\frac{\text{1}}{\text{2}}\) = \(\frac{\text{1}}{\text{16}}\) of the original sample.
a decimal - \(\frac{\text{1}}{\text{16}}\) = 0.0625 of the original sample
This could then be incorporated into other data.
So, if the half-life is two days, four half-lives is 8 days.
Question
If a sample with a half-life of 2 days has a count rate of 3,200 Bq at the start, what is its count rate after 8 days?
If a sample has a count rate of 3,200 Bq at the start, what is its count rate after 8 days?
8 days = 4 half lives.
After 4 half lives the activity remaining would be \(\frac{\text{1}}{\text{2}}\) x \(\frac{\text{1}}{\text{2}}\) x \(\frac{\text{1}}{\text{2}}\) x \(\frac{\text{1}}{\text{2}}\) = \(\frac{\text{1}}{\text{16}}\)
\(\frac{\text{1}}{\text{16}}\) of 3,200 Bq = 200 Bq.
The count rate after 8 days is 200 Bq.
Question
The half-life of cobalt-60 is 5 years. If there are 100 g of cobalt-60 in a sample, how much will be left after 15 years?
15 years is three half-lives so the fraction remaining would be \(\frac{\text{1}}{\text{2}}\) x \(\frac{\text{1}}{\text{2}}\) x \(\frac{\text{1}}{\text{2}}\) = \(\frac{\text{1}}{\text{8}}\)
How much do you know about radioactive decay and half-life?
More on Unit 1: Atomic and nuclear physics
Find out more by working through a topic
- count4 of 6
- count5 of 6
- count6 of 6
- count1 of 6